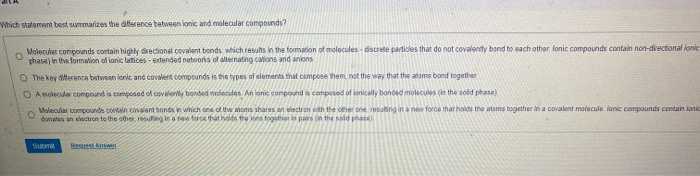
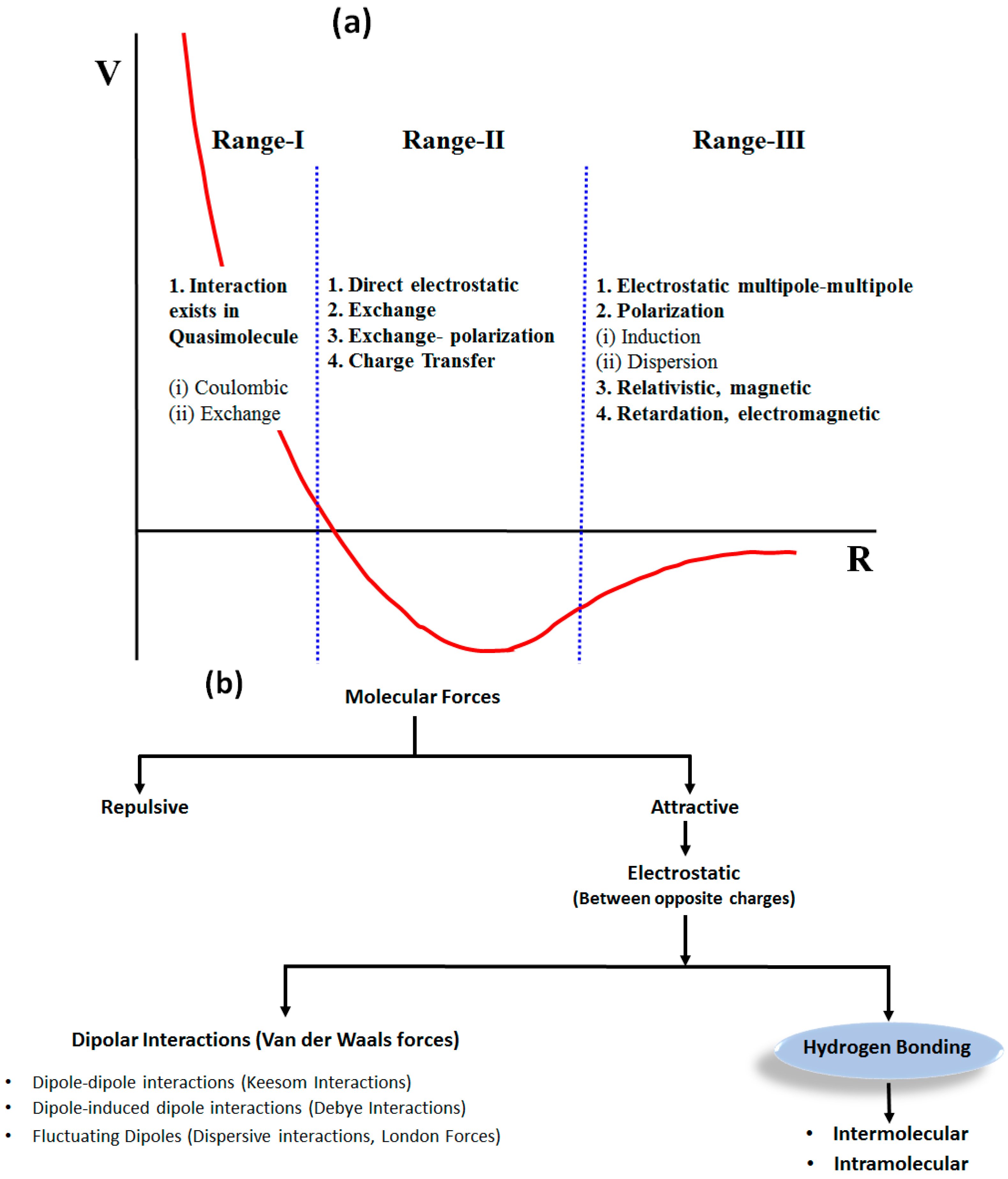
Which Statement Best Summarizes The Difference Between Ionic And Molecular Compounds?
Which statement best summarizes the difference between ionic and molecular compounds?. Ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds from through the sharing of electrons between their outer shells. AMolecular compounds contain two elements and ionic compounds contain three or more elements. Main Differences Between Ionic vs Molecular Compounds.
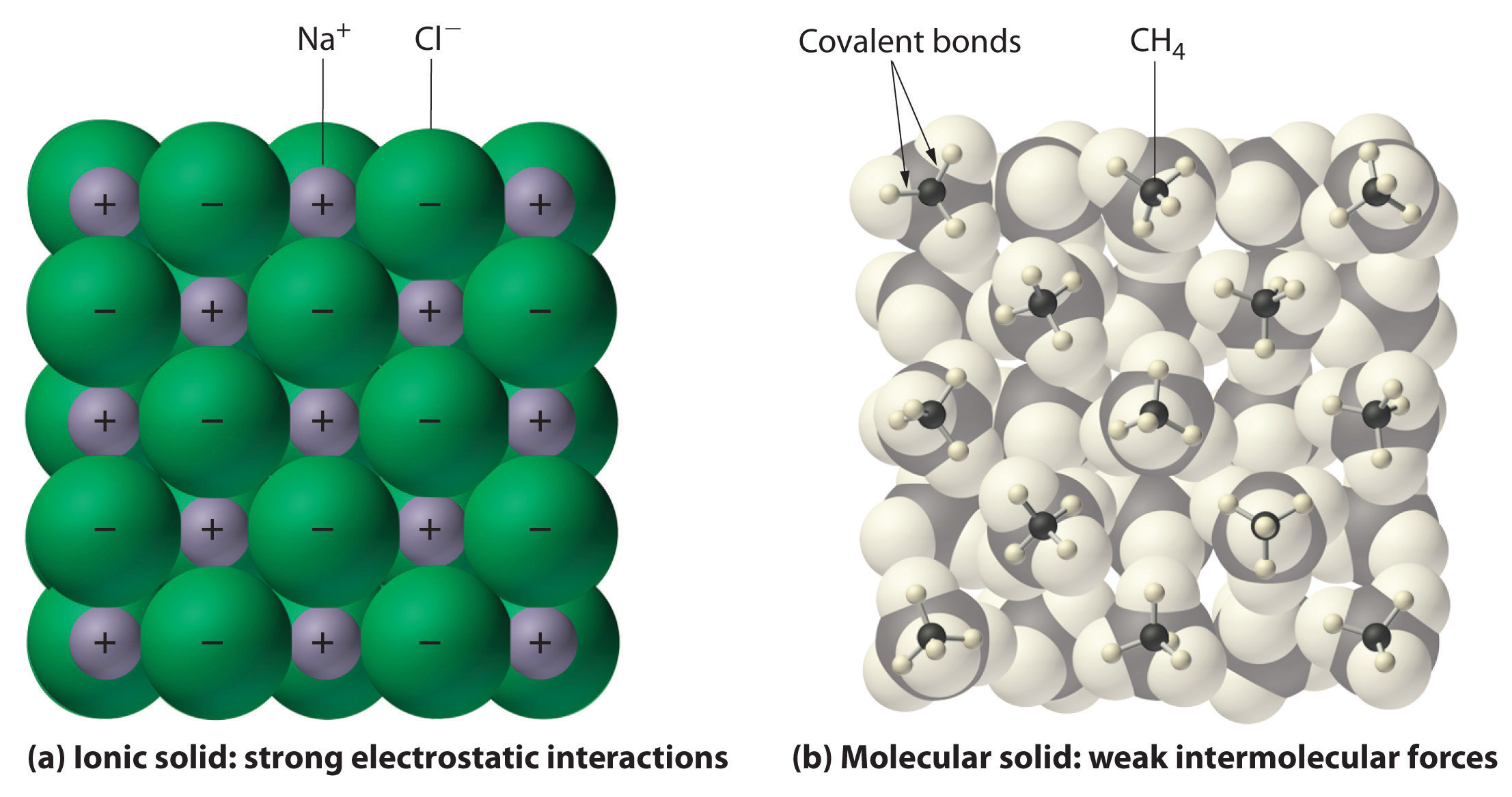
An ionic compound is composed of ionically bonded molecules in the solid phase. NaCl Sodium Chloride KBr Potassium Bromide CaI_2 Calcium Iodide For Transition. CMolecular compounds possess a molecular structural unit and ionic.
An ionic compound is composed of ionically bonded molecules in the solid phase. CMolecular compounds contain covalent bonds in which one. A Molecular compounds contain highly directional covalent bonds which results in the formation of molecules-discrete particles that do not covalently bond to.
A Molecular compounds contain highly directional covalent bonds which results in the formation of molecules-discrete particles that do not covalently bond to each other. Which statement best summarizes the difference between ionic and molecular compounds. This will serve as a comprehensive but quick glance that.
Molecular Compounds are formed due to covalent bond between elements while Ionic Compound is formed due to electrostatic force of attraction known as Ionic bond. CMolecular compounds contain covalent bonds in which one of the atoms shares an electron with the other one resulting in a new force that holds the atoms together in a covalent molecule. An ionic bond is formed between two oppositely charged ions by means of electrostatic attraction.
B The key difference between ionic and covalent compounds is the types of elements that compose them not the way that the atoms bond together. Molecular compounds typically form between a metal and a nonmetal while ionic compounds typically form between nonmetals. The naming of ionic compounds is dependent upon the type of ionic molecule formed from alkali metals alkaline earth metals or transition metals.
The ionic compounds are linked by ionic bonds while the molecular compounds are covalently bonded. Molecular compounds are pure substances formed when atoms are linked together by sharing of electrons while ionic compounds are formed due to the transfer of electrons.
CMolecular compounds contain covalent bonds in which one.
BIonic compounds contain oxygen and molecular compounds do not. Which statement best summarizes the difference between ionic and molecular compounds. Which state best summarizes the difference between lonic and molecular compounds. This will serve as a comprehensive but quick glance that. Which statement best summarizes the difference between ionic and molecular compounds. Hence the key difference between ionic and molecular compounds is that the ionic compounds have electrostatic attraction forces between cations and anions whereas the molecular compounds have only covalent chemical bonds between the atoms. Molecular compounds result from the transfer of electrons between atoms to form ions while ionic compounds result from the sharing of electrons between neutral atoms. Ionic compounds have ionic bonds while molecular compounds have covalent bonds. Molecular compounds typically form between a metal and a nonmetal while ionic compounds typically form between nonmetals.
Ionic compounds are made of ionic bonds and molecular compounds are made of covalent bonds. Molecular compounds are made due to covalent bonding while ionic compounds are made due to ionic bonding. CMolecular compounds contain covalent bonds in which one. Main Differences Between Ionic vs Molecular Compounds. Which state best summarizes the difference between lonic and molecular compounds. A Molecular compounds contain highly directional covalent bonds which results in the formation of molecules-discrete particles that do not covalently bond to. Molecular compounds result from the transfer of electrons between atoms to form ions while ionic compounds result from the sharing of electrons between neutral atoms.




















:max_bytes(150000):strip_icc()/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)



Post a Comment for "Which Statement Best Summarizes The Difference Between Ionic And Molecular Compounds?"